1. Process route
① The synthesis process of silicon powder and hydrogen chloride: Dry silicon powder and hydrogen chloride gas react in a high-temperature and high-pressure synthesis furnace to generate trichlorosilane containing gas, which is condensed through an initial cooling and cryogenic device to obtain a crude product mainly composed of trichlorosilane. The uncondensed exhaust gas is further condensed to recover trichlorosilane, and the remaining exhaust gas is continuously separated and purified through the "adsorption regeneration" process, and then treated again through alkaline washing; The crude product is distilled to obtain trichlorosilane product. The reaction equation is:
Main reaction: 3HCl+Si = SiHCl3+H2
Side reactions: 2HCl+Si = SiH2Cl2, 4HCl+Si = SiCl4+2H2
② Silane coupling agent is obtained by adding trichlorosilane and unsaturated olefins with reactive groups under the catalysis of platinum chlorotic acid, and then undergoing alcoholysis. According to their different central atoms, they are mainly divided into silane, titanate, aluminate ester, etc.
2. Process flow
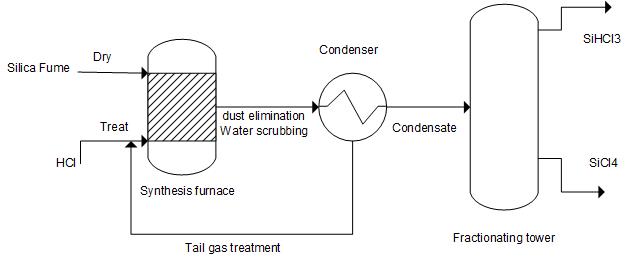
3. Technical advantages
The trichlorosilane technology owned by Shandong Jereh Catech Technology Co., Ltd. is currently the mainstream technology for producing trichlorosilane. This technology can consume a large amount of hydrogen chloride gas and solve the problem of by-product hydrochloric acid restricting the balance of chlor alkali production; At the same time, in addition to developing towards the single/polycrystalline silicon industry, trichlorosilane can also produce silane coupling agents by matching the chloropropene production technology owned by Kaitai Technology, which can achieve overall design, reduce investment, and improve returns.
4. Downstream market direction
① Trichlorosilane → Polycrystalline silicon → Photovoltaics, semiconductors, metal ceramics, etc;
② Trichlorosilane → silane coupling agent → surface treatment, inorganic filling plastics, sealants, thickeners, etc;
③ Trichlorosilane → pharmaceutical industry;
④ Tetrachlorosilicon → organosilicon.






 中文
中文


